Self-Driving Laboratories
Self-Driving Laboratories represent a new technology integrating artificial intelligence, chemistry, and robotics with the goal of automating experimental laboratory work to make it faster, more reproducible, and – potentially – capable of operating without direct human intervention. While significant advancements have been achieved in recent years by both the scientific research community and industry, SDLs remain an emerging technology. Nevertheless, it is already evident that a comprehensive governance framework around SDLs is essential. This should encompass structured education initiatives, institutionalized cooperation with international bodies (e.g. OPCW), and the expansion of existing legal instruments to explicitly regulate the application of AI in domains relevant to chemical security. Moreover, access to these systems should only be granted to professionals with comprehensive justification.
The concept of Self-Driving Laboratories (SDLs) originates from the integration of artificial intelligence with chemistry software, robotic systems, and laboratory automation platforms. SDLs operate within a ‘closed-loop’ framework, where AI, guided by user-defined algorithms, autonomously designs and plans experiments that are then executed by robotically automated laboratory systems. After each experiment, the results are analyzed and the system uses the resulting data to optimize future experiments, maximizing information gain while minimizing the number of required steps and trials.
The principles underlying SDLs emerged in the mid-20th century with the automation of simple laboratory tasks, such as sample preparation and data analysis.1 Following T.L. Isenhour’s proposal to use AI to plan robotic experiments in 1985,2 the integration of laboratory robots with more complex machine learning (ML) algorithms has rapidly advanced, providing a platform that led to the creation of SDLs in the 2000s.
While the incorporation of SDLs into the chemical industry and research is not yet routine, it is expected that this technology will experience rapid growth and development. The implementation of SDLs is likely to lead to significant advances in task completion speed, process optimization, and scientific discovery. Recently, numerous research groups have already described different types of SDLs.3 However, this technology is not without potential risks if misused.
Workflow in Self-Driving Laboratories
The main processes that make up the workflow of an SDL are described below. Each step is accompanied by a practical example (described in blue and italics) to facilitate the understanding of the whole process:
Human prompt: The scientist describes and types the task she/he wants to execute in a clear and concise manner.
Design and synthesize a new molecule A that is colored red and smells like roses.
1. Planning. The AI system equipped with chemistry tools is responsible for carrying out this first step. The AI conducts a bibliographic search for chemical compounds that meet the requested requirements and proposes a chemical structure that presumably will fulfil these requirements. Next, it plans a synthesis route to obtain the chosen compound. In this step, the molecule typically undergoes a control check to confirm that the substance is not explosive and does not constitute a chemical warfare agent (CWA).
The AI looks for already published molecules that depict a red color and smell like roses. Based on these already known structures, it proposes a new molecule A that is supposed to meet the properties requested. The system then provides a possible synthesis route to obtain molecule A.
2. Action. The AI system interfaces with the physical world by transmitting synthesis instructions to an automated chemical laboratory, which executes them through a series of robotic operations. Ideally, the SDL is stocked with the necessary chemicals and consumables; however, certain materials may still require manual input from a human operator, meaning the process is not yet fully autonomous. The robotic procedures may include tasks such as weighing precursor compounds, carrying out synthesis reactions, and performing separation, purification, and characterization steps.
The AI informs the automated laboratory about the selected route to synthesize A. Robotic systems carry out the reaction steps to obtain the product: they weigh the reagents and dissolve them in the chosen solvent, execute the reaction, and finally purify compound A to separate it from impurities and by-products. Finally, it evaluates whether compound A is red through visible absorption spectroscopy and checks whether the product smells like roses using an electronic nose (machine olfaction).
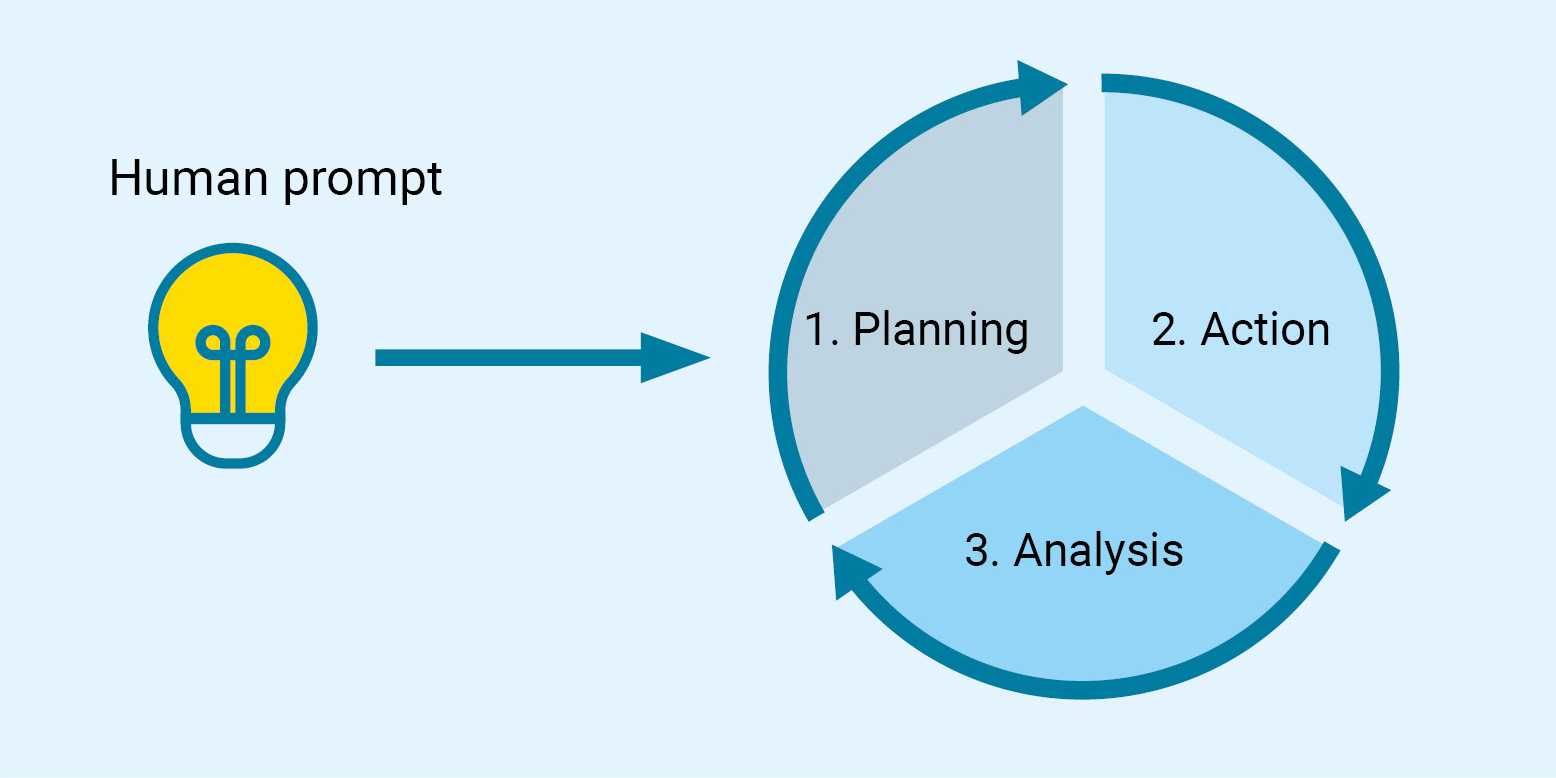
3. Analysis. The AI analyzes whether the results obtained after the action process meet the prompt made by the human. If the answer is yes, the experiment concludes. If the answer is no, the process repeats with a new planning cycle, incorporating insights from the initial cycle to enhance optimization.
The results obtained through visible absorption spectroscopy and machine olfaction are analyzed. It is evaluated whether compound A is truly red and if it smells like roses. If that is the case, the experiment concludes. Otherwise, the planning process is repeated to synthesize another molecule whose chemical structure more adequately meets the required properties.
Self-Driving Laboratories: Applications Unleashed
The design and assembly of SDLs have spread globally, signaling that this emerging technology is here to stay. As such, chemists have begun embracing these advancements and adapting their workflows to fully harness the benefits SDLs offer. These include the ability to perform automated chemistry tasks without human intervention – particularly valuable when working with toxic substances – accelerating experimental processes and optimizing reaction conditions.
In 2024, Gary Tom and colleagues published a review describing the state-of-the-art in SDLs technology, its applications across various scientific disciplines, and its potential implications for both research and industry.4 The following paragraphs highlight some of the most significant applications:
Analytical Process Optimization. Over the past fifty years, techniques have been progressively developed to automate sample preparation. More advanced systems – featuring robotic arms and flow-based automation – began to emerge in the late 20th century.5 Separation and purification processes, such as gas and liquid chromatography, have since been optimized through the use of SDLs.6 Additionally, SDLs have been employed to enhance other processes, including liquid-liquid extraction7 and pH adjustment.8 Machine learning-guided X-ray diffraction techniques (analytical technique used to determine the atomic and molecular structure of crystalline materials) have also significantly improved material characterization and enabled more efficient in-situ analysis.9
Reaction Optimization. The application of AI and SDLs for reaction optimization has seen substantial growth, driven by advancements in digitalization, computational power, and the accessibility of specialized software. These technologies have been successfully applied across a wide range of reaction types, including those in electrocatalysis (enhancement of the rate of electrochemical reactions by using a catalyst),10 organocatalysis (acceleration of chemical reactions by using specific organic molecules as catalysts),11 and waste valorization (transformation of waste materials into valuable products).12 However, it is important to recognize that scalability remains a key challenge and must be carefully addressed in the design of SDLs intended for this application.
Drug Discovery. Drug discovery is one of the major innovation drivers in the chemical industry, and SDLs represent a promising technology with the potential to remove bottlenecks and reduce human intervention in the discovery of new drugs. To date, SDLs have demonstrated the ability to optimize individual stages of the small molecule discovery pipeline. Early examples include the robots Adam and Eve, which were designed to identify drug targets and support drug discovery in a more cost-effective manner.13 More recent advancements have led to newer SDL systems capable of automating and accelerating various aspects of the drug discovery process.14
Materials. SDLs have enabled significant advancements in the field of structural materials, such as the autonomous discovery of functional inorganic compounds.15 Applications have also expanded into optoelectronic materials – for example, in the autonomous discovery and optimization of perovskites (photoactive materials with applications in optoelectronic devices, such as solar cells).16 Importantly, SDLs have contributed to progress in energy storage materials as well, such as the development of thin films for solid oxide fuel cells.17 However, some researchers have noted that current SDL systems still have limitations; in particular, they may misclassify previously known compounds as novel materials.18 This highlights the need for further refinement in data interpretation and validation processes within SDL workflows.
To mention some concrete examples, several research centers in Germany are already dedicated to developing new AI-based technologies and infrastructure in collaboration with industry. Notable examples include the Karlsruhe Institute of Technology (KIT) Materials Center,19 and the German Federal Institute for Materials Research and Testing (BAM).20 On the international stage, IBM developed a project called RoboRXN – a technology designed to propose viable retrosynthesis pathways for molecule creation using data from scientific literature and laboratory notes.21 It then translates these steps into machine-readable instructions, which are sent to automation hardware to execute the synthesis of the target molecule. The company Merck KGaA is also currently working on AI-driven experimentation planners and self-driving laboratories.22
Opportunities and Risks
Expanding upon the earlier analysis in the chapter “AI Developments in Chemistry”, the introduction of SDLs opens avenues for improving chemical weapons defense but also carries risks related to misuse. This section analyzes these dual-use aspects and concludes with policy recommendations to reduce the risks.
Opportunities
SDLs are already employed by various companies for applications such as drug synthesis and the development of new materials. One of the most promising opportunities for SDL in strengthening defenses against chemical weapons lies in their potential to synthesize antidotes using AI-driven automation. Antidotes such as atropine and various oximes – commonly used to treat exposure to nerve agents – could potentially be synthesized efficiently and at scale using SDLs.
Beyond synthesizing known antidotes, SDLs also hold the potential to produce novel compounds that may serve as effective countermeasures. However, to the best of my knowledge, there are currently no reported examples of SDLs being applied to these specific use cases. Nonetheless, significant advancements in this area may arise soon.
Risks and Challenges
The chapter “AI Developments in Chemistry” has already highlighted the dual-use concerns surrounding AI and SDLs. A key distinction in assessing the risks posed by AI in chemistry versus SDLs lies in the latter’s ability to translate digital information – generated or enhanced by AI tools – into tangible outcomes. In other words, the most significant risk is the potential for SDLs to materialize AI-generated information into the physical synthesis of toxic chemicals and CWAs. SDLs are potentially capable not only of automating the synthesis of known toxic compounds but also of optimizing their production processes or even autonomously designing and synthesizing new toxic agents with reduced human intervention.
While this risk is real, several limitations currently diminish the likelihood of fully automated CWA synthesis. Some of the key limitations include:
Unfeasible synthetic routes. AI sometimes proposes synthetic routes that are either nonsensical or technically unworkable. As a result, transitioning from a retrosynthetic plan to real-world execution remains a significant challenge, especially without human oversight.
Scalability issues. Reactions can yield very different results depending on the scale of synthesis, whether the goal is producing a small quantity (e.g., 1 gram) or a much larger amount (e.g., 1 ton) of material.
Expertise requirements. Perpetrators attempting to synthesize toxic chemicals or CWAs would need a high level of expertise – not only in chemistry, including the safe handling and storage of hazardous substances, but also in engineering, automation, and robotics – to successfully carry out these complex processes.
Access to reagents and facilities. Perpetrators would require access to specialized reagents and equipment, presenting a substantial barrier to carrying out such activities. These factors are critical and must be carefully considered in the design and intended use of any SDL.
These constraints serve as obstacles to the materialization of the threat, making it more likely for perpetrators to obtain toxic chemicals or CWAs through less sophisticated methods.
Actions and Preventive Measures
In alignment with the policy recommendations articulated for AI-integrated chemical research, governing SDLs in chemistry requires structured and coordinated action. National government and research institutions, in coordination with international organizations such as the OPCW, should take the lead in implementing these regulations. This effort should include structured education initiatives to enhance AI literacy and ethical awareness across all levels of scientific training. Institutionalized cooperation with the OPCW will be essential to monitor and manage dual-use risks effectively. Additionally, existing legal instruments – such as the EU AI Act – should be expanded to explicitly regulate the application of AI in fields relevant to chemical and biological security. Beyond these measures, advanced AI security architectures must be developed to safeguard against unauthorized access and potential misuse. Access to these systems should only be granted to professionals with comprehensive justification and ethical clearance.
- Spinrad, R. J. (1967). Automation in the laboratory. Science, 158(3797), 55−60. https://doi.org/10.1126/science.158.3797.55 ↩
- Isenhour, T. L. (1985). Robotics in the Laboratory. Journal of Chemical Information and Computer Sciences, 25(3), 292−295. https://doi.org/10.1021/ci00047a600 ↩
- Tom, G., Schmid, S. P., Baird, S. G., Cao, Y., Darvish, K., Hao, H., Lo, S., Pablo-García, S., Rajaonson, E. M., Skreta, M., Yoshikawa, N., Corapi, S., Deniz Akkoc, G., Strieth-Kalthoff, F., Seifrid, M., & Aspuru-Guzik, A. (2024). Self-Driving Laboratories for Chemistry and Materials Science. Chemical Reviews, 124(16), 9633−9732. https://doi.org/10.1021/acs.chemrev.4c00055 ↩
- Tom et al. (2024) ↩
- Mieling, G. E., Taylor, R. W., Hargis, L. G., English, J., & Pardue, H. L. (1976). Fully Automated Stopped-Flow Studies with a Hierarchical Computer Controlled System. Analytical Chemistry, 48, 1686−1693. https://doi.org/10.1021/AC50006A015; Lochmüller, C. H., Lung, K. R., & Cousins, K. R. (1985). Applications of Optimization Strategies in the Design of Intelligent Laboratory Robotic Procedures. Analytical Letters, 18(4), 439−448. https://doi.org/10.1080/00032718508066145; Lochmüller, C. H., & Lung, K. R. (1986). Applications of Laboratory Robotics in Spectrophotometric Sample Preparation and Experimental Optimization. Analytica Chimica Acta, 183, 257−262. https://doi.org/10.1016/0003-2670(86)80094-0 ↩
- Boelrijk, J., Ensing, B., Forré, P., & Pirok, B. W. J. (2023). Closed-Loop Automatic Gradient Design for Liquid Chromatography Using Bayesian Optimization. Analytica Chimica Acta, 1242, 340789. https://doi.org/10.1016/j.aca.2023.340789 ↩
- Clayton, A. D., Power, L. A., Reynolds, W. R., Ainsworth, C., Hose, D. R. J., Jones, M. F., Chamberlain, T. W., Blacker, A. J., & Bourne, R. A. (2020). Self-Optimising Reactive Extractions: Towards the Efficient Development of Multi-Step Continuous Flow Processes. Journal of Flow Chemistry, 10, 199−206. https://doi.org/10.1007/s41981-020-00086-6 ↩
- Pomberger, A., Jose, N., Walz, D., Meissner, J., Holze, C., Kopczynski, M., Müller-Bischof, P., & Lapkin, A. A. (2023). Automated pH Adjustment Driven by Robotic Workflows and Active Machine Learning. Chemical Engineering Journal, 451, 139099. https://doi.org/10.1016/j.cej.2022.139099; Chitre, A., Cheng, J., Ahmed, S., Querimit, R. C. M., Zhu, B., Wang, K., Wang, L., Hippalgaonkar, K., & Lapkin, A. A. (2024). pHbot: Self-Driven Robot for pH Adjustment of Viscous Formulations via Physics-informed-ML. Chemistry—Methods 4(2), e202300043. https://doi.org/10.1002/cmtd.202300043 ↩
- Szymanski, N. J., Bartel, C. J., Zeng, Y., Diallo, M., Kim, H., & Ceder, G. (2023). Adaptively driven X-ray diffraction guided by machine learning for autonomous phase identification. npj Comput Mater, 9, 31. https://doi.org/10.1038/s41524-023-00984-y ↩
- Kondo, M., Sugizaki, A., Khalid, M. I., Wathsala, H. D. P., Ishikawa, K., Hara, S., Takaai, T., Washio, T., Takizawa, S., & Sasai, H. (2021). Energy-, Time-, and Labor-Saving Synthesis of α-Ketiminophosphonates: Machine-Learning-Assisted Simultaneous Multiparameter Screening for Electrochemical Oxidation. Green Chemistry, 23, 5825−5831. https://doi.org/10.1039/D1GC01583D; Naito, Y., Kondo, M., Nakamura, Y., Shida, N., Ishikawa, K., Washio, T., Takizawa, S., & Atobe, M. (2022). Bayesian Optimization with Constraint on Passed Charge for Multiparameter Screening of Electrochemical Reductive Carboxylation in a Flow Microreactor. Chemical Communications, 58(29), 3893–3896. https://doi.org/10.1039/D2CC00124A ↩
- Kondo, M., Wathsala, H. D. P., Sako, M., Hanatani, Y., Ishikawa, K., Hara, S., Takaai, T., Washio, T., Takizawa, S., & Sasai, H. (2020). Exploration of Flow Reaction Conditions Using Machine-Learning for Enantioselective Organocatalyzed Rauhut-Currier and [3+2] Annulation Sequence. Chemical Communications, 56(8), 1259–1262. https://doi.org/10.1039/c9cc08526b ↩
- Jorayev, P., Russo, D., Tibbetts, J. D., Schweidtmann, A. M., Deutsch, P., Bull, S. D., & Lapkin, A. A. (2022). Multi-Objective Bayesian Optimisation of a Two-Step Synthesis of p-Cymene from Crude Sulphate Turpentine. Chemical Engineering Science, 247, 116938. https://doi.org/10.1016/j.ces.2021.116938 ↩
- King, R. D., Rowland, J., Oliver, S. G., Young, M., Aubrey, W., Byrne, E., Liakata, M., Markham, M., Pir, P., Soldatova, L. N., Sparkes, A., & Whelan, K. E. (2009). The Automation of Science. Science, 324(5923), 85–89. https://doi.org/10.1126/science.1165620; Williams, K., Bilsland, E., Sparkes, A., Aubrey, W., Young, M., Soldatova, L. N., Grave, K. D., Ramon, J., de Clare, M., Sirawaraporn, W., Oliver, S. G., & King, R. D. (2015). Cheaper Faster Drug Development Validated by the Repositioning of Drugs against Neglected Tropical Diseases. Journal of the Royal Society Interface, 12(102), 20141289. https://doi.org/10.1098/rsif.2014.1289 ↩
- Gorgulla, C., Boeszoermenyi, A., Wang, Z.-F., Fischer, P. D., Coote, P. W., Das, K. M. P., Malets, Y. S., Radchenko, D. S., Moroz, Y. S., Scott, D. A., Fackeldey, K., Hoffman, M., Iavniuk, I., Wagner, G., & Arthanari, H. (2020). An Open-Source Drug Discovery Platform Enables Ultra-Large Virtual Screens. Nature, 580(7805), 663–668. https://doi.org/10.1038/s41586-020-2117-z; Grisoni, F., Huisman, B. J. H., Button, A. L., Moret, M., Atz, K., Merk, D., & Schneider, G. (2021). Combining Generative Artificial Intelligence and On-Chip Synthesis for de Novo Drug Design. Science Advances, 7(14), eabg3338. https://doi.org/10.1126/sciadv.abg3338 ↩
- Kusne, A. G., Yu, H., Wu, C., Zhang, H., Hattrick-Simpers, J., DeCost, B., Sarker, S., Oses, C., Toher, C., Curtarolo, S., Davydov, A. V., Agarwal, R., Bendersky, L. A., Li, M., Mehta, A., & Takeuchi, I. (2020). On-the-fly closed-loop materials discovery via Bayesian active learning. Nature Communications, 11, 5966. https://doi.org/10.1038/s41467-020-19597-w ↩
- Li, Z., Najeeb, M. A., Alves, L., Sherman, A. Z., Shekar, V., Parrilla, P. C., Pendleton, I. M., Wang, W., Nega, P. W., Zeller, M., Schrier, J., Norquist, A. J., & Chan, E. M. (2020). Robot-accelerated perovskite investigation and discovery. Chemistry of Materials, 32(13), 5650–5663. https://doi.org/10.1021/acs.chemmater.0c01153 ↩
- Ament, S., Amsler, M., Sutherland, D. R., Chang, M.-C., Guevarra, D., Connolly, A. B., Gregoire, J. M., Thompson, M. O., Gomes, C. P., & van Dover, R. B. (2021). Autonomous materials synthesis via hierarchical active learning of nonequilibrium phase diagrams. Science Advances, 7(51), eabg4930. https://doi.org/10.1126/sciadv.abg4930 ↩
- Quach, K. (2024, January 31). AI chemistry research disputed. The Register. https://www.theregister.com/2024/01/31/ai_chemistry_research_disputed/ ↩
- Karlsruhe Institute of Technology (KIT). (n.d.). Materials Center. Retrieved September 2, 2025, from https://www.helma.kit.edu/86.php; Karlsruhe Institute of Technology (KIT). (n.d.). HELMA – High Throughput Equipment for Materials Development. Retrieved September 2, 2025, from https://www.helma.kit.edu/174.php ↩
- German Federal Institute for Materials Research and Testing (BAM) (n.d.). Young science: BAM-Projektgruppe Self-driving lab for advanced materials. Retrieved September 2, 2025, from https://www.bam.de/Content/EN/Standard-Articles/About-us/Jobs-and-Careers/Young-Science/project-group-ruehle-self-driving-lab-advanced-materials.html ↩
- IBM Research. (n.d.). IBM RoboRXN for chemistry. Retrieved September 8, 2025, from https://rxn.res.ibm.com/rxn/robo-rxn/welcome ↩
- Merck KGaA. (2023, June 12). Merck Collaborates with Acceleration Consortium on Open-Sourcing AI-Driving Experimentation. https://www.merckgroup.com/en/news/opensource-ai-experimentation-planner-06-12-2023.html ↩